2021 AFPM Summit Virtual Edition: The changing face of cooling water corrosion control
JAMES GREEN and JEFFREY ZURLO, SUEZ – Water Technologies & Solutions
According to the United Nations, 20% of all water use in the world is used in industrial processes; this figure increases to as high as 80% in industrialized nations.1 Removing heat from critical processes using once-through systems and cooling towers is a significant source of industrial water use. With the demand for water accelerating globally, significant emphasis has been placed on developing technologies that can reduce freshwater demand and increase water recycling.
While significant progress has been made, until recently, there have been limited advancements in technologies that reduce the environmental impact of concentrated discharge water streams, which upset the delicate balance of nutrients in the environments downstream of industrial processes. More than a decade of fundamental research has revealed a novel understanding of the chemistry in cooling water systems and led to advancements in chemical treatment design.
Applying engineered passivation films in aqueous systems provides corrosion protection in a different way than phosphate- or zinc-based systems, while simultaneously eliminating excess biological nutrients—such as phosphorus, heavy metals like zinc (which is an EPA priority pollutant) and hazardous acids—that cause strain on the cooling systems themselves and waste treatment operations. Additionally, this can improve operator safety, increase production rates and further safely concentrate and reuse water in cooling systems.
Challenges. Phosphate-based deposition and corrosion inhibition have been the mainstay technology used for industrial cooling water systems since the 1970s, which saw the elimination of hexavalent chromium. Various forms of phosphate-based programs protect the operating assets of production facilities from failure while enabling those facilities to reduce water usage by 75%–90%; however, they bring challenges.
Advanced polymer dispersants in the cooling water are required to avoid phosphate-related deposition, flow restriction and subsequent under-deposit corrosion. Significant improvements in dispersant technologies, such as dedicated terpolymer-based industry-leading stress tolerant polymer (STP) technology combined with adjunct polymers like alkaline-enhanced chemistry (AEC), lead the way toward reducing phosphate levels by as much as 60%. However, even the relatively small amount of remaining phosphorus in concentrated cooling water discharge streams can result in negative consequences. Further, a delicate polymer to phosphorus balance is required at all times to avoid episodic phosphate deposition, such that control can be difficult to effectively maintain.
Deposition control is not the only issue with using phosphorus. It also has potentially significant negative impacts on downstream environments and ecology. Phosphorus is a limiting nutrient in biological growth, such that excess can result in the additional growth of undesirable biological organisms (FIG. 1). This issue is highlighted in the North American Great Lakes area and Southeast U.S., where there is frequently excessive algae growth. It has been found that 1 lb of phosphorus can generate up to 500 lb of wet algae. Excess algae can lead to dissolved oxygen reduction in the discharge bodies of water, clogging of waterways, and death of marine life. Inside the fence, algae growth can directly impact the ability of a production plant to clean up its water prior to discharge into the environment, which can result in fines and potential loss of production. With such a significant impact on the environment, regulating authorities are, or are in the process of, restricting phosphorus discharge limits from facilities. These discharge levels are so low that the use of phosphate-based materials in cooling water systems has become impractical at effective concentrations.
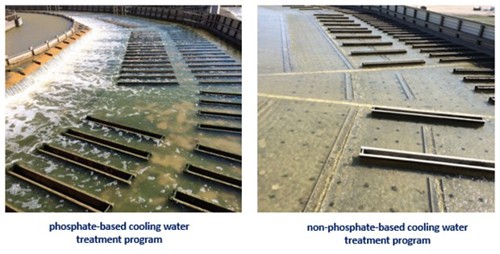
FIG. 1. Compare a phosphate-based cooling water treatment program to a non-phosphate-based program. Challenges with algae growth and control disappear.
To support the changing needs of the industry as outlined above (i.e., reduce phosphorus in cooling systems, improve deposition issues, reduce algae and discharge issues, and improve the environmental profile), water treatment companies have developed and introduced several non-phosphorus solutions. The following will outline the three most common alternatives and their strengths/weaknesses: zinc, tin and engineered films.
Examining alternatives. The most common approach is to utilize zinc and a carboxylic acid polymer to eliminate the need to use phosphorus. Initially invented more than 30 years ago, zinc is a well-known and commonly used corrosion inhibitor. Unfortunately, zinc can also cause deposition issues and is an EPA priority pollutant deemed harmful to the environment. Overall, this approach works well operationally, but significant environmental drawbacks remain.
In the last few years, it has become common for many companies to utilize stannous chloride (tin) in combination with polyaspartic, glucaric or saccharic acid. Tin can improve corrosion control vs. an untreated system, but only in systems that do not use an oxidizer for microbial control, like hypochlorite or bromine. Oxidizers are commonly fed continuously to industrial cooling water systems to control biological growth due to their effective performance and low cost. Tin materials have multiple oxidation states and in a cooling water system where oxidizers are present, the tin is quickly oxidized to an insoluble form and then precipitates in the bulk water, rendering it useless for corrosion control. In addition, tin has a solubility constant (KSP) similar to calcium phosphate salts, meaning it can cause deposition and failures just like a phosphate-based material. The final nail in the coffin for tin materials is that they can cause direct, galvanic corrosion in production assets like heat exchangers. Many companies have attempted to use carboxylic acids to sequester the tin and keep it soluble. While this works well in a storage tank of the chemical product, upon introducing the product to the cooling water system, the carboxylic acid releases the tin, where it is affected by oxidizers and system metals as described above.
The final option covered is to use new technologies based on engineered films. Researchers at SUEZ threw out the fundamental understanding of corrosion control that the industry believed in for more than 40 years and approached the problem differently. Instead of developing anodic or cathodic corrosion inhibitors to prevent the formation of an active corrosion cell, the team redefined the known mechanisms for corrosion control by leveraging a series of techniques to understand every layer of a corrosion-inhibiting, passivation film < 150-nm thick on a metal surface. The team then spent more than a decade developing methods and chemistries to engineer a robust, protective film in an aqueous solution that doesn’t inhibit heat transfer and is thinner than previous phosphate-based technology.
SUEZ’s E.C.O.Film™, or Engineered Carboxylate Oxide (E.C.O.), technology allows customers to meet or exceed changing environmental regulations and take significant steps towards reaching their sustainability goals. Our non-phosphorus technology (may contain trace amounts) helps to reduce toxicity to aquatic life, minimize harmful algae growth and algae blooms, and increase water reuse while delivering the same performance and protection standards that cooling water system operators have come to expect from traditional chemistries (FIG. 2).

FIG. 2. Tin vs. E.C.O.Film performance.
E.C.O.Film uses polymeric technology based solely on carbon-, hydrogen- and oxygen- (CHO) containing polymers. In a cooling water system, the CHO technology primes the metal surface and aids in promoting a passivated metal oxide layer before helping to protect the metal oxide with a protective, dynamic, heterogeneous capping matrix layer (FIG. 3). The film formation takes minutes to hours, not days or weeks, and is self-limiting in thickness. This means that the protective film will never grow to a point where deposition could cause production problems. Instead, it stays 20 nm–50 nm thick.
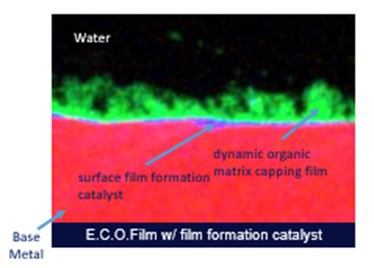
FIG. 3. E.C.O.Film with surface film formation catalyst (SFFC).
In some applications, a patented surface film formation catalyst (SFFC) enhances corrosion protection. This catalyst exclusively targets the metal surface (it is not found in the capping matrix) and helps develop a stronger passivated metal-oxide layer in corrosive water conditions. Typically fed at 92% lower levels than zinc and 96% lower levels than tin, the SFFC is an excellent choice for a non-phosphorus, environmentally conscious approach to treatment. In some applications, it has been documented that asset life (time to failure) can be 10–20 times longer due to the improved corrosion protection of E.C.O.Film (FIG. 4). Unlike tin technology, E.C.O.Film technology is halogen/oxidizer stable, so, it will continue to work and provide protection against harmful corrosion and deposition, regardless of how much hypochlorite or oxidizer is fed to control biological growth.

E.C.O.Film allows production facilities to minimize phosphorus contributions to outfalls, preventing harmful algae growth and detrimental eutrophication of freshwater resources. It can help facilities meet or exceed new and changing phosphorus discharge regulations, and prevent costly deposition associated with phosphate-based materials. Overall, facilities can be a better partner in their community, improve their environmental and brand image, and meet their long-term sustainability goals.
NOTES
1 https://www.un.org/waterforlifedecade/green_economy_2011/pdf/info_brief_water_and_industry_eng.pdf
ABOUT THE AUTHORS
Jim Green serves as a Global Marketing Leader for SUEZ – Water Technologies & Solutions based in Trevose, Pennsylvania, where he focuses on new product development, growth programs and value communication as part of the strategic marketing team. He is a graduate of Principia College and has more than 25 years of experience in industrial water treatment. In the water treatment industry, he has held positions as a Technical Sales Engineer, Global Product Director, Technical Advisor and Vice President across multiple international water and service companies. Mr. Green has authored and presented multiple papers on water treatment subjects for industry organizations, conferences and publications. He is passionate about practical environmental conservation, developing new technologies and optimizing resource use, process and organizational efficiency.

Jeffrey A. Zurlo is the Strategic Marketing and Commercialization Lead for SUEZ – Water Technologies & Solutions. He has more than 30 years of industry experience across several hydrocarbon processing technologies at both facility and corporate level positions in process engineering, technical sales and services, subject matter expertise and strategic marketing. Prior to joining SUEZ – Water Technologies & Solutions in 1997, Mr. Zurlo held process engineering positions at Sunoco Inc., Koch Refining and Hercules Inc. He holds a BE (ChE) degree from Stevens Institute of Technology.







Comments